Which of These Best Describes Sublimation
Conditions the best way to describe sublimation. Condensation is the transition from gas to liquid as in the condensation of steam to liquid water.
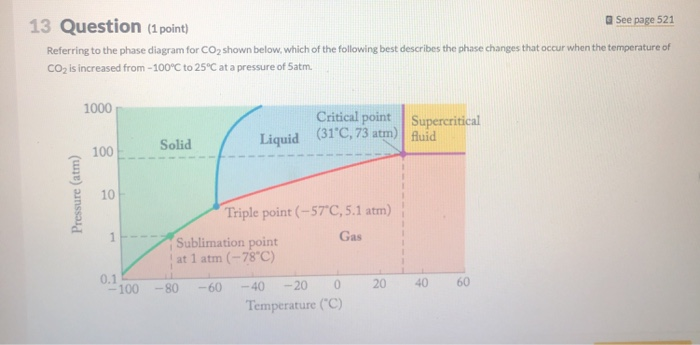
Solved 13 Question 1 Point A See Page 521 Referring To The Chegg Com
A Molecules of the solute are attracted to one another more than the solvent.

. The phenomenon is the result. What is happening to the energy level of a substance during sublimation. A solid melting to a liquid B.
Which sentence describes an example of sublimation. Which of the following statements accurately describes energy during the phase change of sublimation. Answer choices It is a chemical reaction because the kinetic energy of the molecules is changing.
Sublimation is the conversation between the solid and the gaseous phases of matter with no intermediate liquid stage. A solid forming a gas. Which of the following best describes what takes place during the solvation process.
The answer is D because its where a solid turns to a gas without going through a liquid phase if that makes sense Send. Which statements best describe how the properties of gases and plasmas differ. Which of these images best represents the conservation of mass.
Any solid-vapour transition is called sublimation. Which of the following best describes sublimation. Sublimation is the transition from the solid to the gas state.
An example is the vaporization of frozen carbon dioxide at ordinary atmospheric pressure and temperature. Which of the following best describes the process of sublimation. The opposite of this process is called deposition.
During sublimation the particles In a solid. Dmitriy789 7 10 months ago. List 2 i Can flow in all directions ii The temperature at which a liquid changes into its gaseous state iii Can have any number of free surfaces iv Gaps between.
B Solvent molecules surround solute molecules and pull them out into the solution. A good example is carbon dioxide ice. Since it has been argued that in these deflagration processes residence.
Which of the following best describes sublimation. Fusion vaporization and sublimation are endothermic processes. Literary translation in particular is relevant to all these sciences audio-visual arts as well as cultural and intellectual studies.
Examples of sublimation are burning of camphor iodine and naphthalene is really evaporation of a solid. A solid forming a gas. One what point on the diagram do the solid liquid and gas phases coexist simultaneously.
The movement of gaseous particles so that they fill the space they occupy D. An interesting one is graphite which sublimates at 3915C 4020C Langes Handbook of Chemistry 15th edition. For those of us interested in the water cycle sublimation is most often used to describe the process of snow and ice changing into water vapour in the air without frist melting into water.
Sublimation is defined as a process in which solid converts into gas directly without converting into liquid. Dew forms on leaves on a cold morning - 18160640. A solid melting to a liquid which then evaporates C.
Alex41 277 10 months ago. The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. C Molecules of the solvent are attracted to one another and exclude solute molecules.
The graph shows the phase diagram of a substance. Sublimation is the direct change from a gas to a solid without entering into the liquid state. Which of these best describes sublimation.
The solid carbon dioxide sublimates and becomes a gas at room temperature. In Psychology sublimation is a mature defense mechanism in which something. Changing from solid to gas.
Other examples include iodine arsenic and naphthalene moth balls. Sublimation is the process in which a substance changes its physical state from solid to gas without entering liquid state. The transition from the gas to the solid.
The best-known example of sublimation is dry ice. Which of these statements describes what happens to molecules of a solid as the temperature is lowered to absolute zero -273 C. Which of the following best describes sublimation.
Gain so much energy they turn to gas withoutbecoming a liquid. Sublimation in physics conversion of a substance from the solid to the gaseous state without its becoming liquid. Which of the following best describes sublimation.
List 1 a Solids b Sublimation c Boiling point d Gases e Intermolecular space. Sublimation of Ammonium Perchlorate1 greatest practical importance.

Chemistry 2005 Released Test 1 How Many Valence Electrons Does A Neutral Atoms Of Silicon Have Ppt Download

Energy Phases Of Matter Equilibrium Review Game Ppt Download
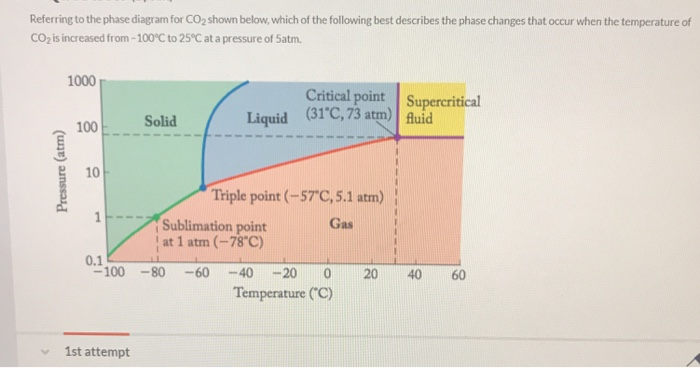
Solved Referring To The Phase Diagram For Co2 Shown Below Chegg Com
Comments
Post a Comment